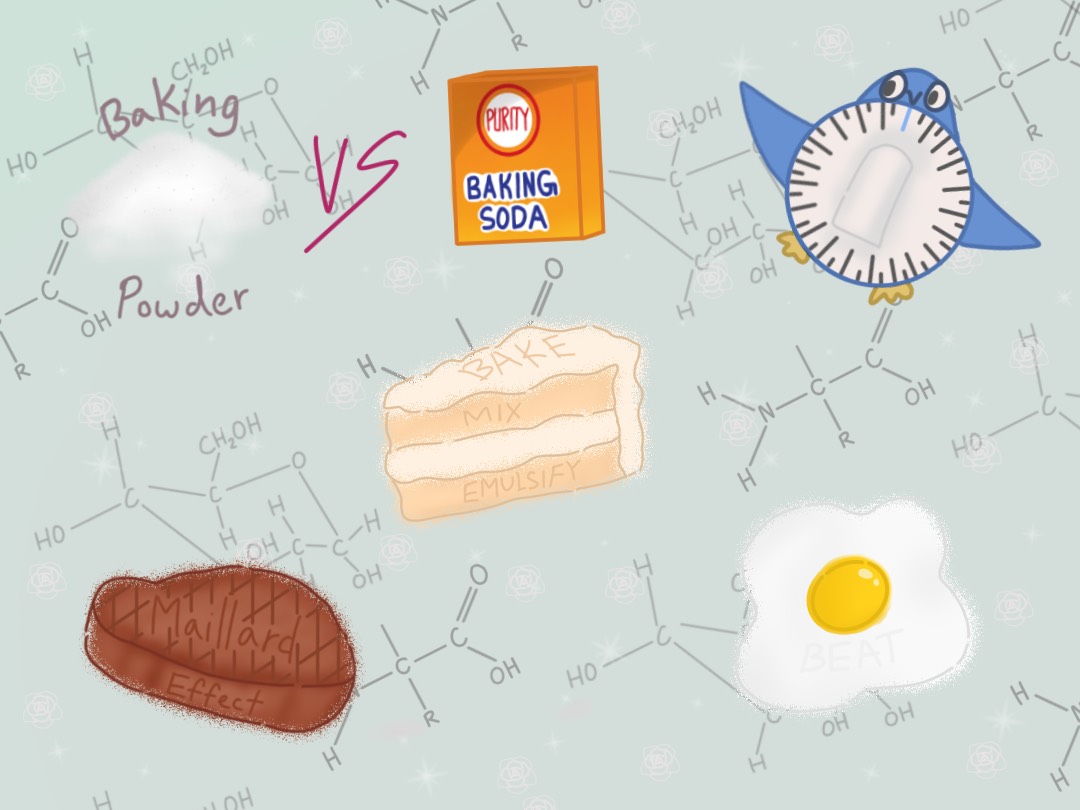
How does one transform eggs, flour, butter, sugar, and a dash of salt into a warm and fluffy cake? Sure, using an oven is essential, but it’s only half the story. The chemical reactions taking place within the batter are the key to baking, and understanding them might make baking a little simpler.
Also known as the browning reaction, the Maillard reaction occurs when reactive “reducing” sugars interact with the amino acids of proteins, forming small molecules that trigger a multitude of other chemical reactions. Reducing sugars are small, simple sugar compounds, such as glucose and fructose. The reaction between the sugars and amino acids results in the golden brown “crust” of so many different foods, including bread, cookies, coffee beans, and meat.
Sophomore Cody Htet believes cooking requires a lot more chemistry than most people think. After cooking for around two years, Htet’s passion for cooking has led him to do some research in the chemistry of cooking.
“Boiling water, melting cheese, grilling steak. All are chemical and physical reactions. In the Maillard reaction, steak becomes brown when cooked because [the protein] myoglobin loses its ability to [bind] with oxygen, and it loses an electron.” Thus, the myoglobin is oxidized, resulting in the crispy brown edges of a perfectly grilled steak.
In order to optimize the golden brown crust on your food, the temperature must around 300℉. Additionally, pH can affect the Maillard reaction. pH is a scale that measures the level of acidity or basicity in a substance. Higher pH, which is more alkaline, increases the rate of the reactions and a lower, more acidic pH slows down the browning process.
Another of the most common ingredients found in the pantry is baking soda. Sodium bicarbonate is a leavening agent that has a relatively high pH of 9, making it very basic. The ingredient reacts with acidity, forming carbon dioxide gas that allows bread to rise in a process known as “chemical leavening.” Baking powder seems almost identical to baking soda, but with a few key differences. Along with sodium bicarbonate, baking powder also contains two acids, which extend the leavening process. One of the acids reacts immediately with the sodium bicarbonate once it becomes wet, activating the chemical reaction immediately. The other acid, usually sodium acid pyrophosphate or sodium aluminum sulfate, needs to be both liquified and placed in a hot temperature. This is why substituting baking soda for baking powder is not recommended. The different amounts of sodium bicarbonate in the agents alter the taste of the final baked good, resulting in a tangy, metallic taste if too much is added.
“I’ve always wondered the difference between baking powder and baking soda,” said senior Matthew Ho. “I never really thought there was a difference, but after doing some research, I’ve learned that the difference is pretty important. A person who didn’t do research on these ingredients could end up substituting one for the other, not knowing that it has a negative impact on their final product.”
While skimming through a recipe for soufflé, one might notice the emphasis on beating the egg whites prior to mixing with other ingredients. This task, especially tedious without a stand mixer, may not seem all that important. What’s the difference between a beaten and unbeaten egg? The secret is in the air bubbles. Egg whites are made of proteins, which are amino acids tangled up into three-dimensional structures, with hydrophilic amino acids surrounding the perimeter and hydrophobic amino acids in the middle, away from the water environment around it. By beating the egg whites, air bubbles begin to form. When a protein is up against an air bubble, the protein starts to change shape and unravel, pushing the hydrophobic amino acids to the air and the hydrophilic away from the bubble. Once the proteins have uncurled, they bind to each other, making them strong enough to hold a shape once the air bubbles have burst. This creates the airy and fluffy texture that makes soufflé and meringues so unique.
Another process done in baking is tempering eggs; this consists of adding small amounts of hot liquid to room-temperature eggs, creating a more stable base for the pastry being made.
Senior Aviya Ramji, an avid baker, explained, “It’s really important to never skip the tempering of egg yolk…because of the characteristics of eggs when it comes in contact with heat, not tempering them first will result in scrambled eggs!”
Tempering eggs helps dilute the proteins, preventing them from unraveling and bunching up like they do in scrambled eggs. By adding hot liquid in controlled amounts, the baker can slowly mix the egg to ensure that the proteins are emulsified in the solution.
While it might not seem all that important, understanding the chemistry in baking is actually quite advantageous.
“Bakers are able to experiment more efficiently when creating new products because [they] know how things react, which is super helpful when pioneering a new dish,” said Ramji. “You can use the knowledge of baking chemistry by being able to discern exactly what’s wrong with a failed product, or being able to add your own twist on a popular dish by adding new ingredients to create a different texture or flavor.”
There are dozens of other chemical reactions that take place when baking. Understanding even a few of them can make baking a much simpler experience, especially when experimenting with new recipes. Hopefully, learning a bit about baking chemistry will help you in the future to make your own pastries and give you a different perspective on the science behind sweet treats.